
The two R groups in the carboxyl region (right) are hydrophobic the two in the amine region (left) are polar.ġ32-2 Amino Acid Sequence & Protein Primary Structureģ.2.1.b Determining Protein Primary Structure - Polypeptide Sequencing The positions of the circled amino acid R groups alternate along the polypeptide backbone. Figure 3.3: The amino and carboxyl ends of a polypeptide define its polarity, with positively charged amino and negatively charged carboxyl ends at physiological pH. A partial polypeptide is shown below in Figure 3.3. You can prove this to yourself by making a short polypeptide with the kind of molecular modeling kit you may have used in a chemistry class! The C-C-N-C-C-N- backbone is the underlying basis of higher orders (or levels) of protein structure.
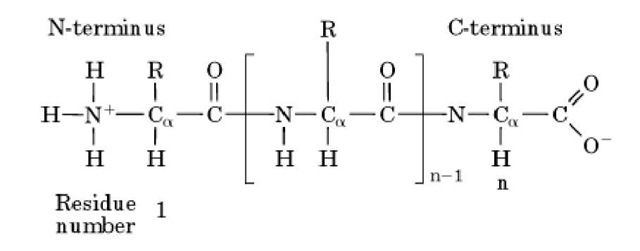
polypeptide backbone because of covalent-bond angles along the backbone. Amino acid side chains end up alternating on opposite sides of a C-C-N-C-C-N.
POLYPEPTIDE BACKBONE FREE
We say that polypeptides have polarity because they have “free” carboxyl ends and free amino ends. Just to make life interesting, L–amino acids are dextrorotary in a polarimeter, making them (d)–amino acids! While both (d)– and (l)–amino acid enantiomers exist in cells, only (d)–(i.e., L–) amino acids (along with glycine) are used by cells to build polypeptides and proteins by the process called translation. Remember, only the lower-case d and l define the optical properties of isomers. Recall that chiral carbons allow for mirror image D and L (or d and l) optical isomers. a Peptide Bond Formation and Polypeptide Primary Structure In all twenty amino acids (except glycine), the \(\alpha\)− carbon is bound to four different groups, making them chiral or optically active.ģ.2.1. All side chains (R groups) are shown at the bottom of each structure, bound to an \(\alpha\) carbon Figure 3.2: Chemical characteristics of the twenty amino acids found in the proteins of cells. The twenty amino acids found in proteins are shown below in Figure 3.2. They break and rearrange between the carboxyl and amino groups of amino acids during linkage formation. The linkages involve multiple covalent bonds. The peptide linkages between amino acids in polypeptides form in condensation reactions in cells during protein synthesis (i.e., translation). Cells use only twenty amino acids to make polypeptides and proteins, although they do use a few additional amino acids for other purposes. The primary structure of a protein simply refers to the amino acid sequence of its polypeptide chain(s).
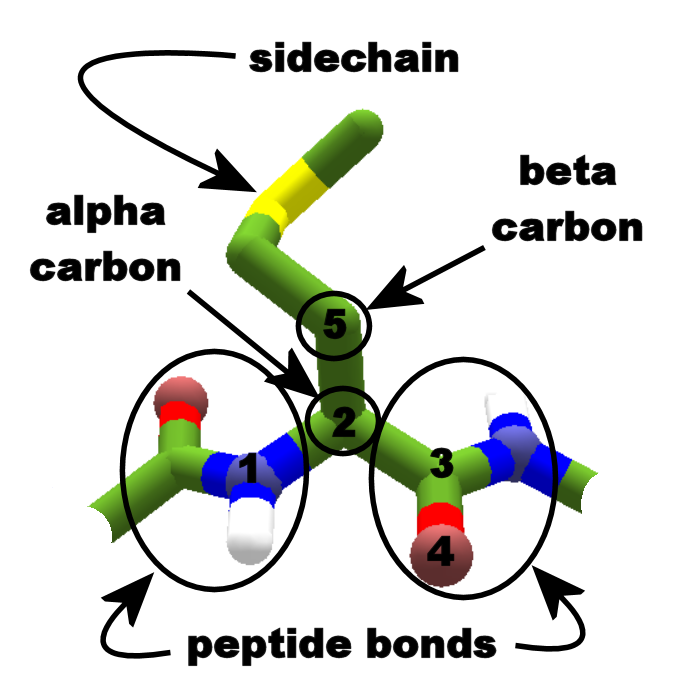
Protein Primary Structure: L-Amino Acids and the -C-C-N- Polypeptide Backbone


 0 kommentar(er)
0 kommentar(er)
